Validierungsservice
Validierung und Qualifizierung von Laborgeräten und Prozessanalysetechnik
Ein wesentlicher Bestandteil von Validierungsprozessen ist die Qualifizierung von Geräten und Systemen. Als Hersteller von Laborgeräten und prozessanalytischen Systemen begegnen wir bei SCHMIDT + HAENSCH der enormen Nachfrage nach Qualifizierungsdienstleistungen mit einem ganzheitlichen Konzept. Sie erhalten eine Dokumentation, die die gesetzlichen Anforderungen und Industriestandards für die Gerätequalifizierung unterstützt.
Unser Qualifizierungsprozess ist so konzipiert, dass Sie dabei Kosten, Ressourcen und Zeit sparen. Unsere Spezialisten haben jahrelange Erfahrung in der Qualifizierung von Messgeräten und kennen die aktuellen regulatorischen Anforderungen der geltenden Richtlinien, Normen und Gesetze. Mit unseren Qualifizierungsdienstleistungen für Messgeräte können Sie sicher sein, dass Ihre Messgeräte gemäß den Spezifikationen des Herstellers und internationalen Standards wie 21 CFR Part 11 Konformität, IQ, OQ, PQ oder IPV und GLP/GMP installiert sind, arbeiten und funktionieren.

Bereits bei der Entwicklung und Herstellung von SCHMIDT + HAENSCH-Geräten wird auf die Einhaltung der Good Manufacturing Practice (GMP) und Good Laboratory Practice (GLP) geachtet. Alle Geräte sind so konzipiert, dass die Konformität mit GLP und GMP über die gesamte Lebensdauer des Gerätes gewährleistet ist. Um diesen Status zu erhalten, werden Service und Support von unseren geschulten Serviceexperten durchgeführt und stets GMP/GLP-konform dokumentiert. Die Standardqualifizierung ist eine GMP/GLP-konforme Qualifizierung von Laborgeräten bei unseren Kunden vor Ort. Die Dokumentation umfasst: alle erforderlichen IQ-, OQ- und PQ-Dienstleistungen in der Arbeitsumgebung unserer Kunden; alle erforderlichen Dokumente, Prüfprotokolle und Zertifikate; Bereitstellung aller für die Qualifizierung erforderlichen zertifizierten Prüfmittel sowie Mess-, Regel- und Spezialwerkzeuge; SOP-Unterstützung auf Basis der Anforderungen des Anwenders und der Messaufgabe; umfassende anwendungsbezogene Schulung des Bedienpersonals.
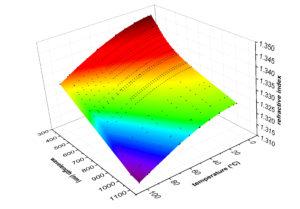
Bei der Installationsqualifizierung (IQ), der Betriebsqualifizierung (OQ) und der Leistungsqualifizierung (PQ) bzw. der Überprüfung der Geräteleistung (IPV) wird überprüft und dokumentiert, ob Ihr Gerät die Leistungsspezifikationen des Herstellers erfüllen kann. Alle Schritte dieses Verfahrens werden von geschulten Ingenieuren durchgeführt.
Die Software-Validierung stellt sicher, dass die Software Ihre kundenspezifischen Anforderungen erfüllt. Die Dokumentation der Softwarevalidierung reduziert den Aufwand für die Integration des neuen Geräts in Ihr System.
Sie benötigen Unterstützung?
Sprechen Sie uns gerne an


